Ion Chromatograph Analysis on Three Samples
Gideon Analytical Laboratories received three samples in polyethylene bottles to develop an analytical procedure for determining chloride using an ion chromatograph. Ion chromatography is a process that allows the separation of ions and polar molecules based on their charge. Using ion chromatography, one can identify concentrations of major anions, including elements like sodium, lithium, calcium, and many others. Aqueous samples are quantitatively measured in the parts-per-million range.
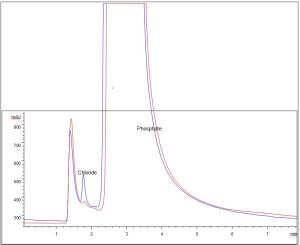
The two solutions were diluted 1:25 with DI water (18.2megaohm) water from a point of use Millipore purification system. The samples were then analyzed by ion chromatography; it was carried out with an instrument that consisted of HP1050 pump and autosampler with a Dionex AMMS suppressor and conductivity detector. Data was captured using an HP Chemstation. Displayed in the table below is the ion chromatograph readings of the respective samples. The picture shows the first sample (in red) at 3.4 PPM and the second sample (in blue) at 35.1 PPM. The results of the ion chromatography revealed the certain presence of chloride; absolute identity was made by spike, subtraction, and standards.
Gideon Analytical Laboratories is a dynamic research laboratory that as the analytical equipment on site for a variety of scientific inquiry. From radiography, to ion chromatography, Gideon Analytical Laboratories has the knowledgeable personnel and scientific research equipment to be a valuable resource for your analytic needs.
Ion Chromatograph Table